- Home
- About
- Practices
Cruise Ship AccidentsCLASS ACTION LAWSUITSMaritimePersonal Injury
- Results
- Blogs/News
- Free Consultation
- 866-597-4529
Aronfeld Trial Lawyers is representing those how have sufferred Heart Problems, Stroke or Blood Clot Complications after using Zeljanz to treat rheumatoid arthritis (RA), psoriatic arthritis (PsA), or ulcerative colitis (UC)
The U.S. Food and Drug Administration (FDA) has announced new, required safety warnings to be added to prescribing information for Xeljanz and Xeljanz XR, including both 5mg twice daily dose and 10mg twice daily dose.
On September 01, 2021, the FDA issued an update to a previous safety communication regarding an increased risk for blood clots, cardiac effects, cancer, and death in patients who take Xeljanz or Xeljanz XR. The new communication indicates that the FDA has required that increased risk warning be added to prescribing information for the medication.
The latest announcement is based on completed results from a clinical safety study which show increased risk for cardiac effects when compared to other TNF inhibitors like Humira.
Xeljanz and Xeljanz XR (tofacitinib) are prescription medications used to treat adults with the following conditions:
An overactive immune system contributes to rheumatoid arthritis, psoriatic arthritis, and ulcerative colitis. Tofacitinib (Xeljanz, Xeljanz XR) works by decreasing the activity of the immune system.
Xeljanz and Xeljanz XR are manufactured by Pfizer Inc., an American multinational pharmaceutical corporation.
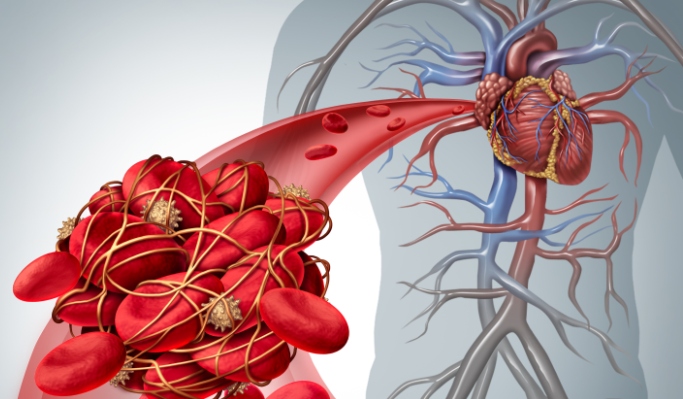
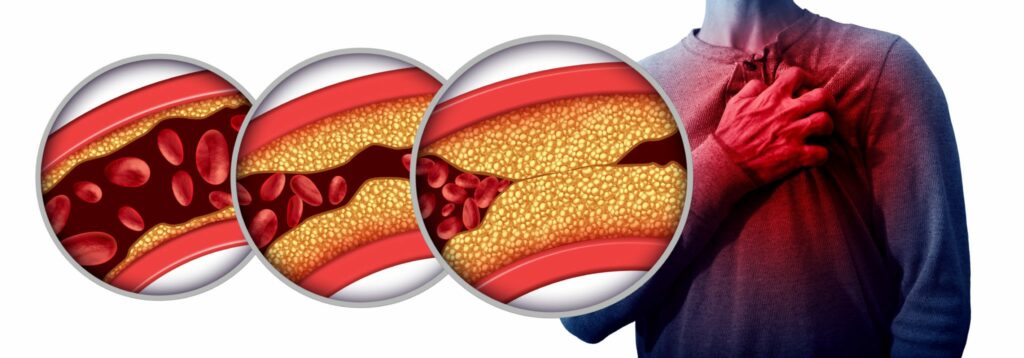
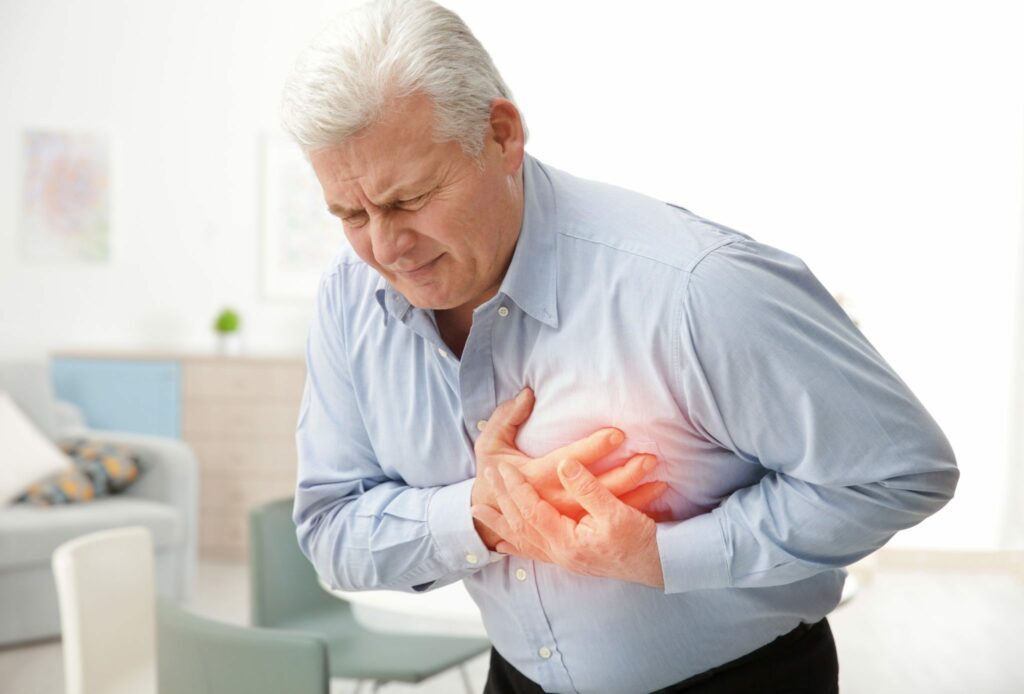
The FDA has linked Xeljanz to following serious side effects: cancer, cardiovascular events, and venous thromboembolism
Xeljanz has been linked to a greater rate of major adverse cardiovascular events, including:
The malignancies most frequently associated with Xeljanz include:
The adverse outcomes most associated with Xeljanz’s risk of blood clots include:
If you or a loved one are suffering from complications after taking Xeljanz or Xeljanz XR, you may be entitled to compensation. Our experienced defective drug and medical device attorneys can help. Click, Chat or call 866-597-4529 24/7 for a free case evaluation.
Aronfeld Trial Lawyers is a firm of high-profile, nationally recognized legal advocates who work for you, our client, never big business. We represent cases resulting in serious injuries in the areas of Cruise Ship Injuries, Wrongful Death, Automobile Accidents, Cycling Accidents, Slip and Fall Incidents, Product Liability, Civil Rights Claims, Workplace Injuries, Maritime Law, Sexual Assault, Medical and Dental Malpractice, and others.
Quick Links
Contact Us
Let’s Work Together
©Aronfeld. 2024 KCoWeb All Rights Reserved